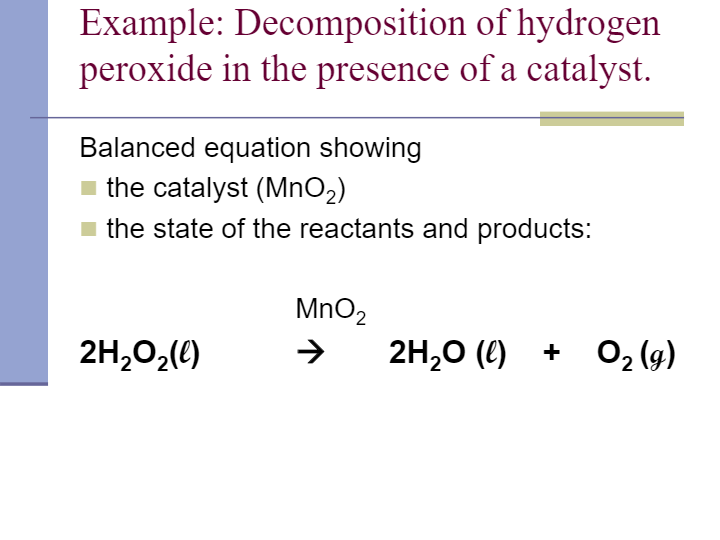
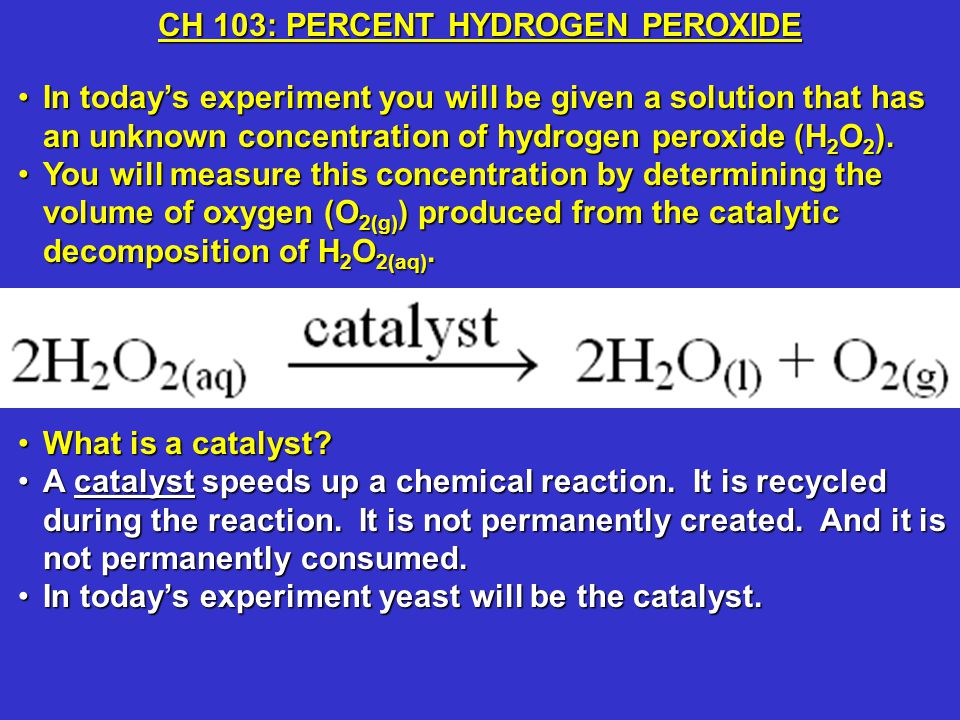
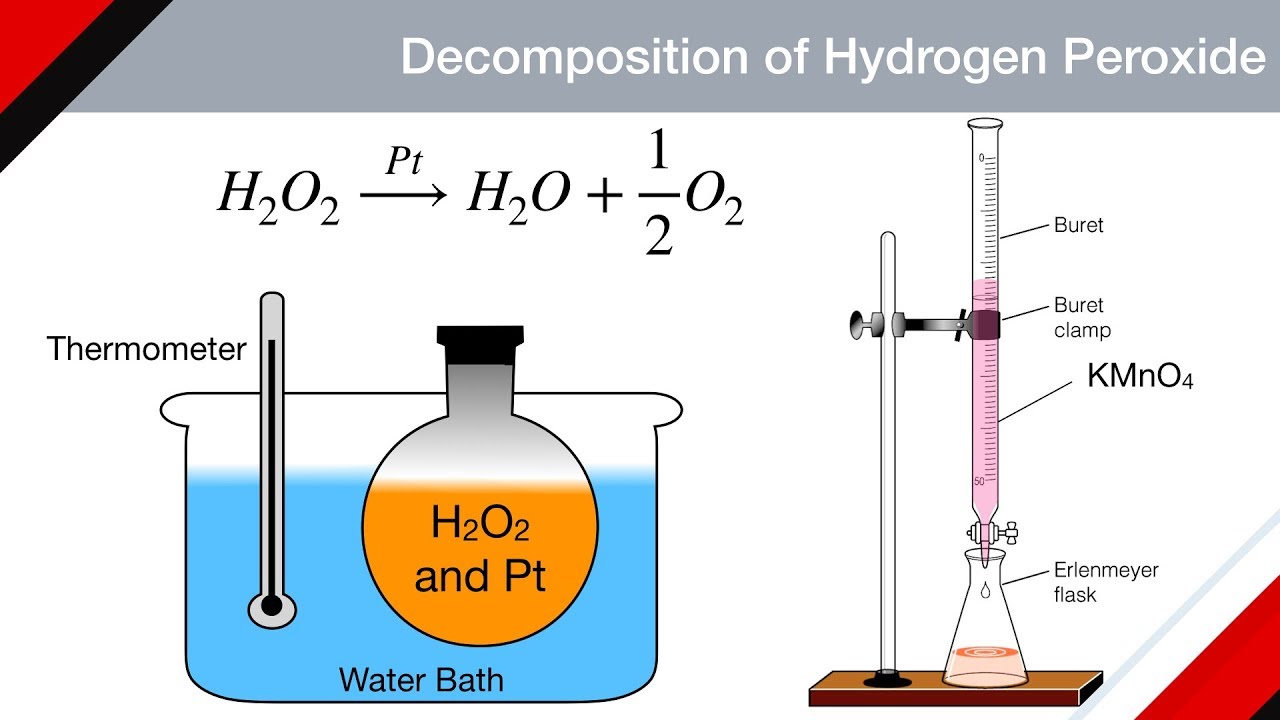
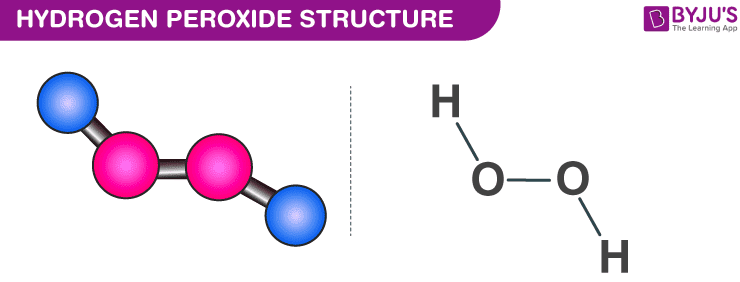
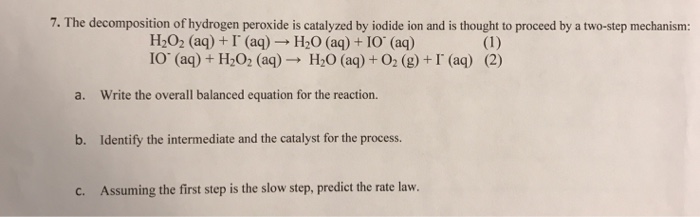
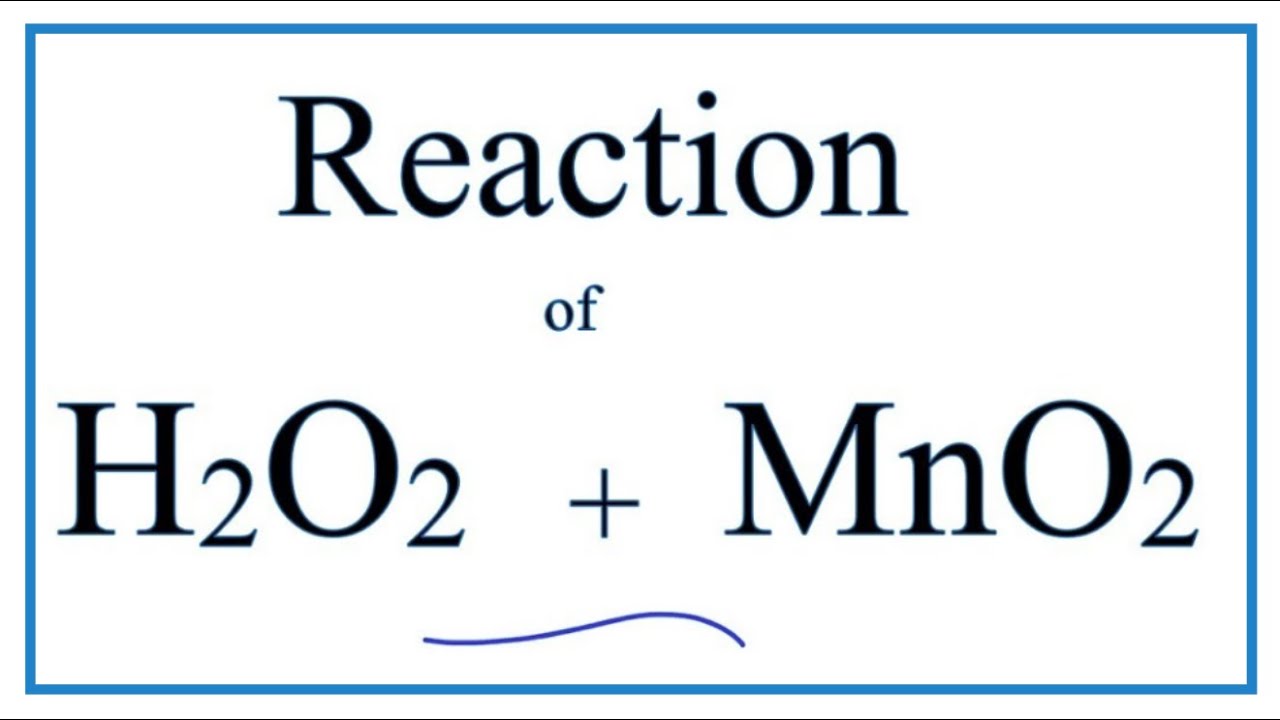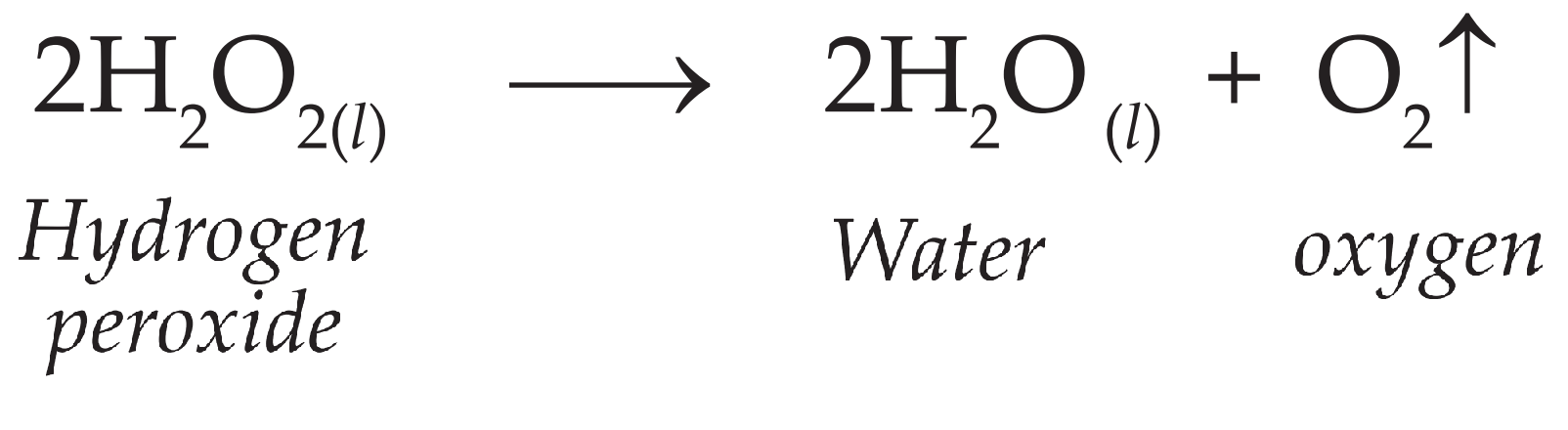OneClass: A proposed mechanism for the decomposition of hydrogen peroxide consists of three elementar...
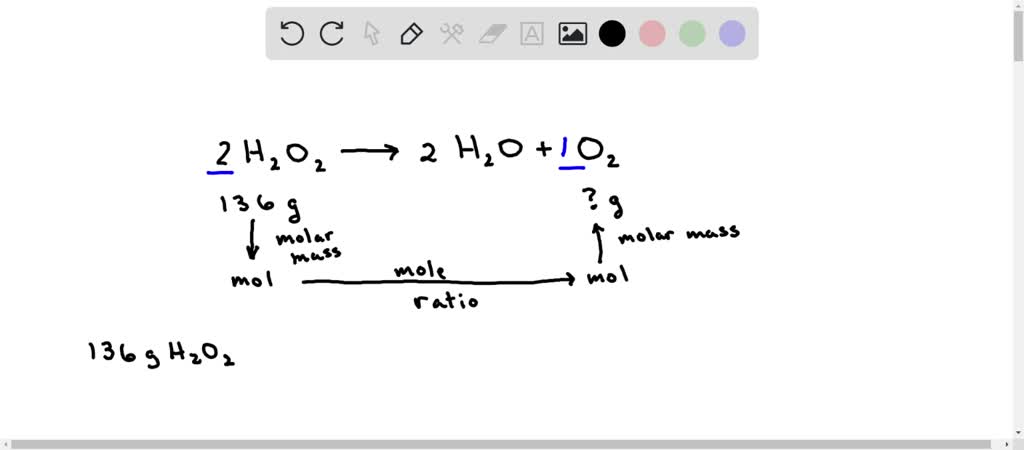
SOLVED: According to the balanced equation below describing the decomposition of hydrogen peroxide to form water and oxygen, if 136 grams of hydrogen peroxide are used, what mass of oxygen gas will
How does a catalyst make Hydrogen Peroxide's decomposition quicker? What is actually happening? | Socratic

Question Video: Using Word Equations to Describe the Decomposition of Hydrogen Peroxide (H2O2) | Nagwa
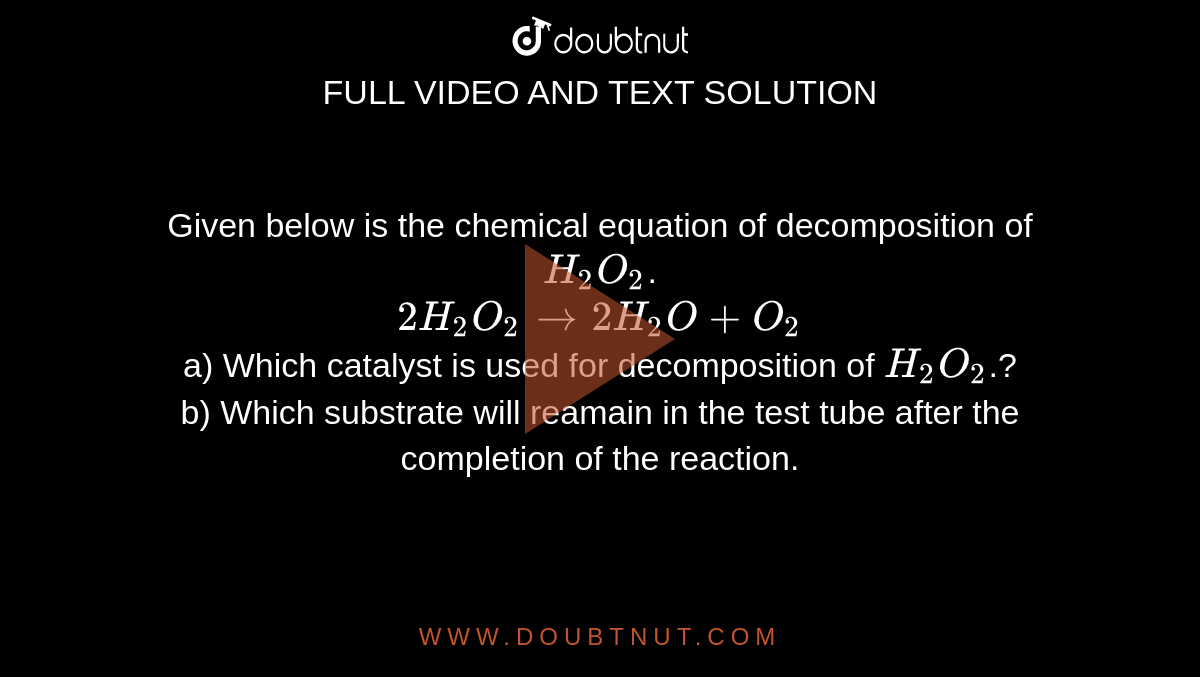
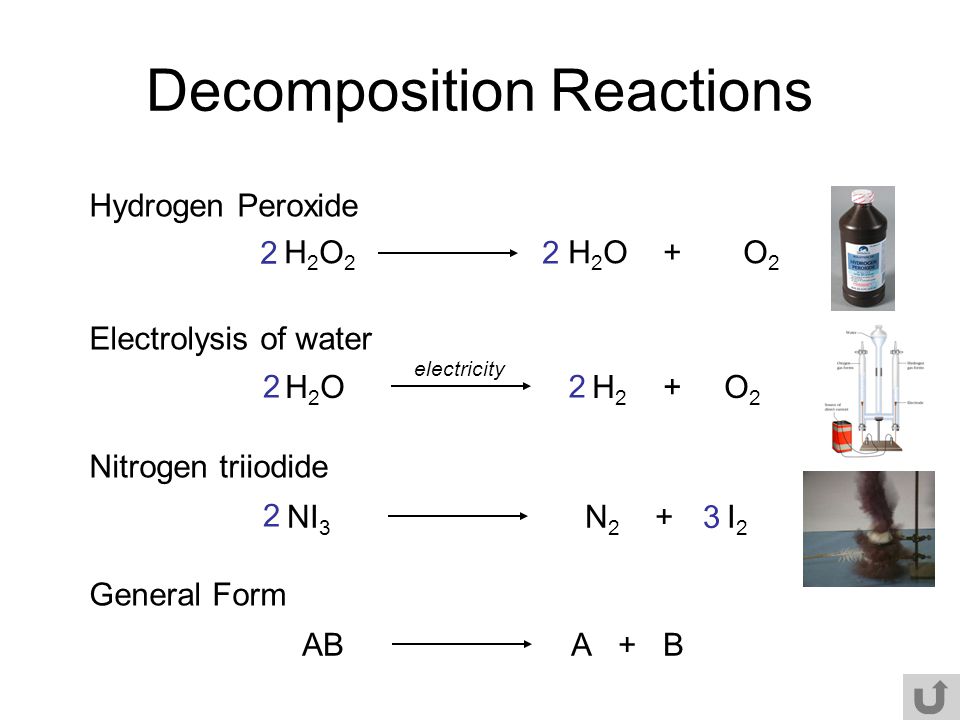
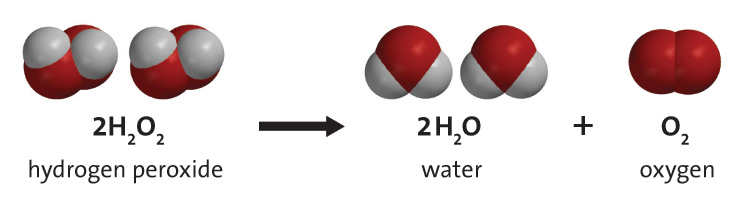
Given below is the chemical equation of decomposition of H2O2. 2H2O2 rarr 2H2O + O2 a) Which catalyst is used for decomposition of H2O2.? b) Which substrate will reamain in the test
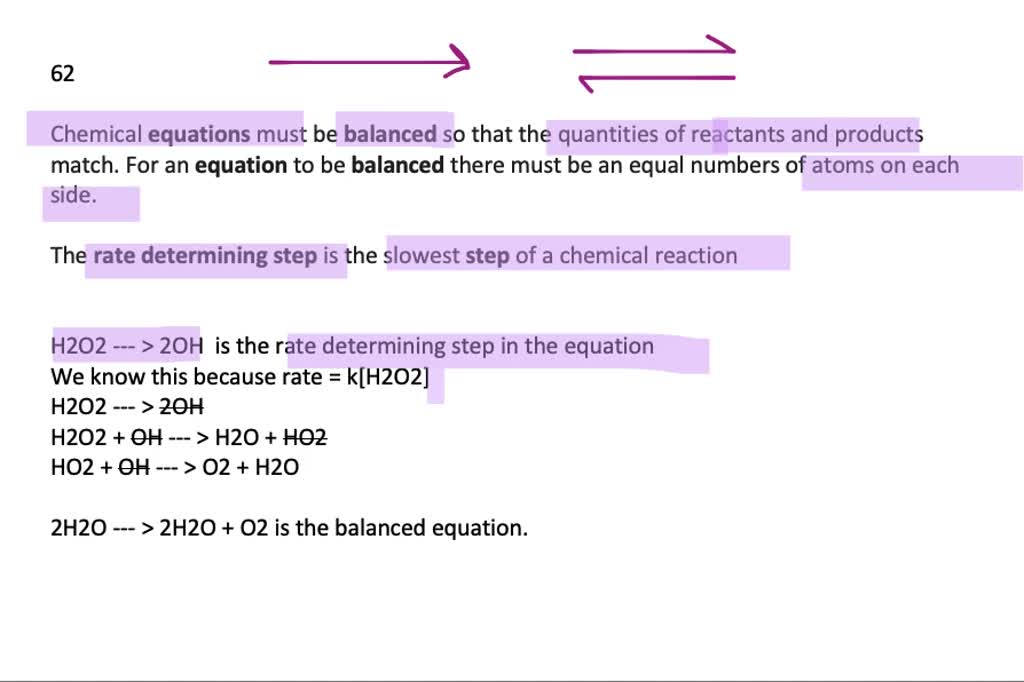
SOLVED:A possible mechanism for the decomposition of hydrogen peroxide is H2O2⟶ 2 OH H2O2+OH⟶H2O+HO2 HO2+OH⟶H2O+O2 Using your results from Exercise 39, specify which step is the rate-determining step. What is the overall

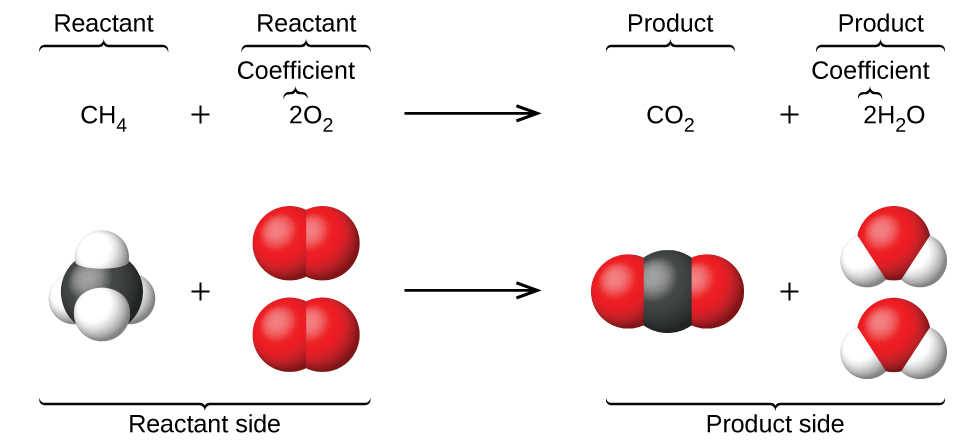
ENTRY QUIZ 1 1.What is chemical reaction? 2.Give an example? 3.What is at the left? 4.What is at the right? 5.What the arrow means? - ppt download
![PDF] Decomposition of hydrogen peroxide - kinetics and review of chosen catalysts | Semantic Scholar PDF] Decomposition of hydrogen peroxide - kinetics and review of chosen catalysts | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f2948b1ccb4f9f878523a741f22c7a299fa747b2/2-Figure1-1.png)